|
|
|
|
|
|
ALL Drugs in this category cause drowsiness, and most can be used to induce different stages of anesthesia, some more quickly than others, and with differing side-effects.
Stages of Anesthesia
These stages occur (when using inhalation anesthesia alone; other drugs added will modify these stages.
Stage 1 (Induction, aka voluntary excitement). Excitement and struggling are common. Usually accompanied by ephinephrine release with associated rise in respiratory rate and heartrate.
Stage 2 (delirium, involuntary excitement). Voluntary centers and loss of consciousness begin. Exaggerated reflexive responses to stimuli are common, as is vomiting (in species that can vomit). Breath holding may occur. Common hazard: self-injury.
Stage 3 General Anesthesia
Plane 1—Light anesthesia. Most reflexes (pedal, corneal, palpebral) are still present.
Plane 2 – Medium anesthesia. Most surgeries are conducted at this level. Muscles are relaxed. Most reflexes (pedal, palpebral, corneal) are absent.
Plane 3—Deep anesthesia. Intercostal muscles are relaxed; ability to maintain respiration is endangered. Pupillary light reflex may be slow or absent.
Plane 4—Too Deep. All muscles, including diaphragm & intercostal muscles, are paralyzed.
Stage 4 Irreversible Anesthesia—respiratory arrest, followed by circulatory collapse. Death within 1-5 minutes.
|
|
The Drunkenness of Noah, Venice, S. Marco, c. 1215 |
|
WHAT wonders does not wine perform?
It discloses secrets, Whom has not a cheerful glass made eloquent! Whom not quite free and easy from pinching poverty? |
quid non ebrietas dissignat ?
operta recludit, fecundi calices quem non fecere disertum? contracta quem non in paupertate solutum? |
Horace Letter 5.1 to Torquatus, (often incorrectly cited as Horace, Odes 3.21, as in e.g. T. Dormandy, The Worst of Evils, p. 18.)
|
|
|
|
ETHANOL |
METHANOL |
|
Produced by yeast-fermentation. |
Produced by destructive distillation |
GIVEN time and sunlight, alcoholic fermentation can occur in any organic potpourri which contains sugar or honey. Much indirect evidence suggests that the product was discovered long before man progressed from food-gathering to settled farming. Even if the taste did not immediately appeal, the happy state engendered may have sent some cave dwellers back for another experimental sip — and then another. Yet the urge remains peculiar to man. As Pliny observed, only the gods and humans drink for reasons other than to quench their thirst. The Worst of Evils, Thomas Donandy, (Yale Univ. Press, 2006) p.16.
THE
SEQUENTIAL
EFFECTS
of ETHANOL
on the HUMAN
BRAIN
see also:
https://pubs.niaaa.nih.gov/publications/arh314/310-339.htm
|
|
From: Goodman and Gilman, The Pharmacological Basis of Therapeutics, Brunton, Chabner, et.al. 12th ed. (McGraw-Hill Medical, New York, 2011). Ch 23 “Ethanol and Methanol”
TOLERANCE,
DEPENDENCE,
and
CHRONIC
ETHANOL
USE
Tolerance is defined as a reduced behavioral or physiological response to the same dose of ethanol,
There is a marked acute tolerance that is detectable soon after administration of ethanol. Acute tolerance can be demonstrated by measuring behavioral impairment at the same BELs (blood ethanol level) on the ascending limb of the absorption phase of the BEL–time curve (minutes after ingestion of alcohol) and on the descending limb of the curve as BELs are lowered by metabolism (one or more hours after ingestion). Behavioral impairment and subjective feelings of intoxication are much greater at a given BEL on the ascending than on the descending limb. [This means that alcohol-induced impairment is greater when measured soon after beginning alcohol consumption than when measured later in the drinking session, even if the BAC is the same at both times]
There also is a chronic tolerance that develops in the long-term heavy drinker. In contrast to acute tolerance, chronic tolerance often has a metabolic component owing to induction of alcohol-metabolizing enzymes.
Physical dependence is demonstrated by the elicitation of a withdrawal syndrome when alcohol consumption is terminated. The symptoms and severity are determined by the amount and duration of alcohol consumption and include[:]
sleep disruption,
autonomic nervous system (sympathetic) activation,
tremors,
and in severe cases, seizures.
In addition, two or more days after withdrawal, some individuals experience delirium tremens, characterized by[:]
hallucinations,
delirium,
fever, and
tachycardia/
Delirium tremens can be fatal. Another aspect of dependence is craving and drug-seeking behavior, often termed psychological dependence.
|
|
PAPAVER SOMNIFERUM - OPIUM POPPY |
|
EVIDENTLY cultivated since prehistoric times, opium, of which the active principle is morphine, is obtained from the dried juice of the scored poppy, papaver somniferum. In the fifth century BC the Greek author Xenophon (c.410 BC) wrote concerning opium (which he called mandragoras):
|
WINE truly ‘moistens the soul’and lulls our griefs to sleep, just as opium (mandragoras) does with men, at the same time awakening kindly feelings as oil quickens a flame. |
τῷ γὰρ ὄντι ὁ οἶνος ἄρδων τὰς ψυχὰς τὰς μὲν λύπας ὥσπερ ὁ μανδραγόρας τοὺς ἀνθρώπους͵ κοιμίζει͵ τὰς δὲ φιλοφροσύνας͵ ὥσπερ ἔλαιον 2.25 φλόγα͵ ἐγείρει. |
Xenophon Symposium 2.24-25
|
|
|
|
MORPHINE |
HEROIN |
|
|
 |
|
CODEINE |
OXYCODONE |
|
|
|
FENTANYL |
MORPHINE, the active principle in opium was identified and purified in 1804 by the German chemist and pharmacist, Friedrich Wilhelm Adan Sertürner. The much more rapidly-acting and addictive drug heroin was created in 1874 by Heinrich Dreser, a professor at Göttingen University, working at the Beyer laboratory in Germany. It was named for the heroic (heroisch) sensation reported by the first human subjects who were given it (several of whom became addicted).
|
|
|
|
OPIUM PARAPHERNALIA |
DERIVATIVES |
OPIOID drugs are used primarily for the treatment of pain . Some of the CNS mechanisms that reduce the perception of pain also produce a state of well-being or euphoria. Thus, opioid drugs also are taken outside medical channels for the purpose of obtaining the effects on mood. This potential for abuse has generated much research on separating the mechanism of analgesia from that of euphoria in the hope of eventually developing a potent analgesic that does not activate the brain reward systems. Although this research has led to advances in understanding the physiology of pain, the standard medications for severe pain remain the derivatives of the opium poppy (opiates) and synthetic drugs that activate the same receptors (opioids). Drugs modeled after the endogenous opioid peptides may one day provide more specific options for the treatment of pain, but none of these currently is available for clinical use.
Analgesic medications that do not act at opioid receptors, such as the nonsteroidal anti-Inflammatory drugs (NSAIDs - aspirin, motrin), have an important role in mild to moderate types of pain,
but for severe pain, the opioid drugs are most effective.
Progress in pain control stems from a greater understanding of the mechanism of tolerance to μ opiate receptor–mediated analgesia, which involves NMDA receptors (Trujillo and Akil, 1991). Experimentally, by combining morphine with dextromethorphan, an NMDA-receptor antagonist, tolerance is impaired and analgesia is enhanced without an increase in the dose of opioid.
The subjective effects of opioid drugs are useful in the management of acute pain. This is particularly true in high-anxiety situations, such as the crushing chest pain of myocardial infarction, when the relaxing, anxiolytic effects complement the analgesia. Normal volunteers with no pain given opioids in the laboratory may report the effects as unpleasant because of side effects such as nausea, vomiting, and sedation.
Patients with pain usually do not develop abuse or addiction problems. Of course, patients receiving opioids over time develop tolerance routinely, and if the medication is stopped abruptly, they will show the signs of an opioid-withdrawal syndrome, the evidence for physical dependence.
Opioids should never be withheld from patients with cancer out of fear of producing addiction. If chronic opioid medication is indicated, it is preferable to prescribe an orally active, slow-onset opioid with a long duration of action. These qualities reduce the likelihood of producing euphoria at onset and withdrawal symptoms as the medication wears off.
In selected patients, methadone is an excellent choice for the management of chronic severe pain. Controlled-release oral morphine (MS CONTIN, others) and controlled-release oxycodone (OXYCONTIN, others) are other possibilities. Rapid-onset, short-duration opioids are excellent for acute short-term use, such as during the postoperative period. As tolerance and physical dependence develop, however, the patient may experience the early symptoms of withdrawal between doses, and during withdrawal, the threshold for pain decreases. Thus, for chronic administration, long-acting opioids are preferred. While methadone is long acting because of its metabolism to active metabolites, the long-acting version of oxycodone has been formulated to release slowly, thereby changing a short-acting opioid into a long-acting one. Unfortunately, this mechanism can be subverted by breaking the tablet and making the full dose of oxycodone immediately available. This has led to diversion of oxycodone to illicit traffic because high-dose oxycodone produces euphoria that is sought by opiate abusers. The diversion of prescription opioids such as oxycodone and hydrocodone to illegal markets has become an important source of opiate abuse in the U.S.
The risk for addiction is highest in patients complaining of pain with no clear physical explanation or in patients with evidence of a chronic, non-life-threatening disorder. Examples are chronic headaches, backaches, abdominal pain, or peripheral neuropathy. Even in these cases, an opioid may be considered as a brief emergency treatment, but long-term treatment with opioids should be used only after other alternatives have been exhausted. In the relatively rare patient who develops abuse, the transition from legitimate use to abuse often begins when the patient returns to his physician earlier than scheduled, asking for a new prescription, or visits emergency rooms of multiple hospitals, complaining of acute pain and asking for an opioid injection.
It has been alleged since 2017 that Oxycontin, billed as providing 12-hour relief, actually fades earlier in many patients. It is further alleged that an 8-hour dosing schedule (not recommended by the manufacturer) increases withdrawal symptoms and increases craving, (L.A. Times July 8, 2018, et.al.)
Heroin is the most frequently abused opiate. There is no legal supply of heroin for clinical use in the U.S. Despite claims that heroin has unique analgesic properties for the treatment of severe pain, double-blind trials have found it to be no more effective than hydromorphone. However, heroin is widely available on the illicit market, and its price dropped sharply in the 1990s, continuing to the present, with purity increased 10-fold.
PREVIOUSLY, street heroin in the U.S. was highly diluted:
Each 100-mg bag of powder had only ~ 4 mg heroin (range: 0-8 mg), and the rest was filler such as quinine.
In the mid-1990s, street heroin reached 45-75% purity in many large cities, with some samples testing as high as 90%.
This increase in purity has led to increased levels of physical dependence among heroin addicts. Users who interrupt regular dosing develop more severe withdrawal symptoms. Whereas heroin previously required intravenous injection, the more potent supplies can be smoked or administered nasally (snorted), making the initiation of heroin use accessible to people who would not insert a needle into their veins.
There is no accurate way to count the number of heroin addicts, but based on extrapolation from overdose deaths, number of applicants for treatment, and number of heroin addicts arrested, the estimates range from 800,000 to 1 million in the U.S.; based on a stratified national sample of adults, -1 in 4 individuals who report any use of heroin become addicted (Anthony et al., 1994). Recent law enforcement actions may presage the emergence of a domestic supply of heroin precursor (raw opium) harvested from Papaver somniferum grown in the Pacific Northwest of the U.S. (Baer, 2009).
TOLERANCE,
DEPENDENCE,
and WITHDRAWAL
Injection of a heroin solution produces a variety of sensations described as
warmth,
taste,
or high
and intense pleasure (“rush”)
often compared with sexual orgasm.
There are some differences among the opioids in their acute effects, with morphine producing more of a histamine-releasing effect and meperidine producing more excitation or confusion. Even experienced opioid addicts, however, cannot distinguish between heroin and hydromorphone in double-blind tests.
Thus, the popularity of heroin may be due to its availability on the illicit market and its rapid onset. After intravenous injection, the effects begin in less than a minute. Heroin has high lipid solubility, crosses the blood-brain barrier quickly, and is deacetylated to the active metabolites 6-monoacetyl morphine and morphine.
After the intense euphoria, which lasts from 45 seconds to several minutes,
there is a period of sedation and tranquility (“on the nod”) lasting up to an hour.
The effects of heroin wear off in 3-5 hours, depending on the dose.
Experienced users may inject two to four times per day.
Thus, the heroin addict is constantly oscillating between being “high” and feeling the sickness of early withdrawal (Figure 24–4).
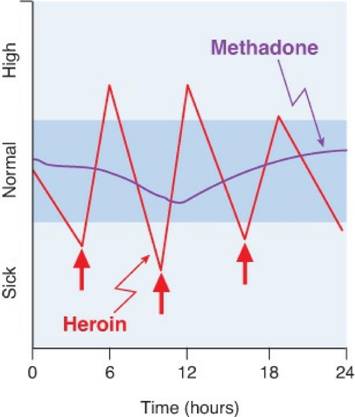 |
Figure 24–4. Differences in responses to heroin and methadone. A person who injects heroin (↑) several times per day oscillates (red line) between being sick and being high. In contrast, the typical methadone patient (purple line) remains in the “normal” range (indicated in blue) with little fluctuation after dosing once per day. The ordinate values represent the subject’s mental and physical state, not plasma levels of the drug.
This produces many problems in the homeostatic systems regulated at least in part by endogenous opioids. For example, the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis are abnormal in heroin addicts. Women on heroin have irregular menses, and men have a variety of sexual performance problems. Mood also is affected. Heroin addicts are relatively docile and compliant after taking heroin, but during withdrawal, they become irritable and aggressive.
Based on patient reports, tolerance develops early to the euphoria-producing effects of opioids. There also is tolerance to the respiratory depressant, analgesic, sedative, and emetic properties. Heroin users tend to increase their daily dose, depending on their financial resources and the availability of the drug. If a supply is available, the dose can be increased progressively 100 times. Even in highly tolerant individuals, the possibility of overdose remains if tolerance is exceeded. Overdose is likely to occur when potency of the street sample is unexpectedly high or when the heroin is mixed with a far more potent opioid, such as fentanyl (SUBLIMAZE, others).
Addiction to heroin or other short-acting opioids produces behavioral disruptions and usually becomes incompatible with a productive life. There is a significant risk for opioid abuse and dependence among physicians and other health care workers who have access to potent opioids, tempting them toward unsupervised experimentation. Physicians often begin by assuming that they can manage their own dose, and they may rationalize their behavior based on the beneficial effects of the drug. Over time, however, the typical unsupervised opioid user loses control, and behavioral changes are observed by family and co-workers. Apart from the behavioral changes and the risk of overdose, especially with very potent opioids, chronic use of opioids is relatively nontoxic.
Opioids frequently are used in combinations with other drugs. A common combination is heroin and cocaine (“speedball”). Users report an improved euphoria because of the combination, and there is evidence of an interaction, because cocaine reduces the signs of opiate withdrawal, and heroin may reduce the irritability seen in chronic cocaine users.
The mortality rate for street heroin users is very high. Early death comes from
involvement in crime to support the habit;
from the uncertain dose, purity, and even identity of what is purchased on the street;
and from serious infections associated with nonsterile drugs and sharing of injection paraphernalia.
Heroin users commonly acquire bacterial infections producing skin abscesses; endocarditis; pulmonary infections, especially tuberculosis; and viral infections producing hepatitis C and acquired immune deficiency syndrome (AIDS).
As with other addictions, the first stage of treatment addresses physical dependence and consists of detoxification (Kosten and O’Conner, 2003). The opioid-withdrawal syndrome (Table 24–7) is very unpleasant but not life-threatening. It begins within 6-12 hours after the last dose of a short-acting opioid and as long as 72-84 hours after a very long-acting opioid medication. Heroin addicts go through early stages of this syndrome frequently when heroin is scarce or expensive. Some therapeutic communities as a matter of policy elect not to treat withdrawal so that the addict can experience the suffering while being given group support. The duration and intensity of the syndrome are related to the clearance of the individual drug. Heroin withdrawal is brief (5-10 days) and intense. Methadone withdrawal is slower in onset and lasts longer. Protracted withdrawal also is likely to be longer with methadone. (See more detailed discussions of protracted withdrawal under “Long-Term Management” later in the chapter.)
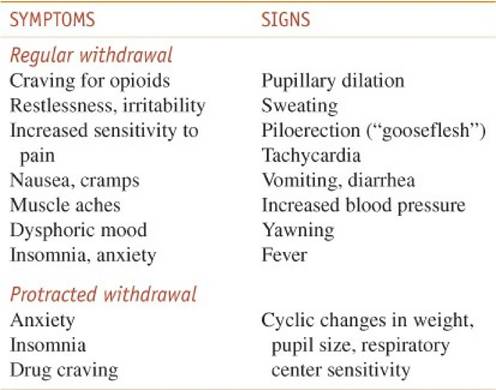 |
Table 24–7 Characteristics of Opioid Withdrawal
Pharmacological Interventions.
Opioid withdrawal signs and symptoms can be treated by three different approaches.
[1] The first and most commonly used approach depends on cross-tolerance and consists of transfer to a prescription opioid medication and then gradual dose reduction. The same principles of detoxification apply as for other types of physical dependence. It is convenient to change the patient from a short-acting opioid such as heroin to a long-acting one such as methadone. Detoxification and subsequent maintenance of opiate dependence with methadone is specifically limited to accredited opioid treatment programs (OTPs) and is regulated by Federal Opioid Treatment Standards. The initial dose of methadone is typically 20-30 mg. This is a test dose to determine the level needed to reduce observed withdrawal symptoms. The first day’s total dose then can be calculated depending on the response and then reduced by 20% per day during the course of detoxification.
[2] A second approach to detoxification involves the use of oral clonidine (CATAPRES, others), a medication approved only for the treatment of hypertension. Clonidine is an α2 adrenergic agonist that decreases adrenergic neurotransmission from the locus ceruleus. Many of the autonomic symptoms of opioid withdrawal such as nausea, vomiting, cramps, sweating, tachycardia, and hypertension result from the loss of opioid suppression of the locus ceruleus system during the abstinence syndrome. Clonidine, acting upon distinct receptors but by cellular mechanisms that mimic opioid effects, can alleviate many of the symptoms of opioid withdrawal, but not the generalized aches and opioid craving. When using clonidine to treat withdrawal, the dose must be titrated according to the stage and severity of withdrawal, beginning with 0.2 mg orally; postural hypotension is commonly a side effect. A similar drug, lofexidine (currently in clinical trials in the U.S.), has greater selectivity for α2A adrenergic receptors and is associated with less of the hypotension that limits the usefulness of clonidine in this setting.
[3] A third method of treating opioid withdrawal involves activation of the endogenous opioid system without medication. The techniques proposed include acupuncture and several methods of CNS activation using transcutaneous electrical stimulation. While attractive theoretically, this has not yet been found to be practical.
[4] Rapid antagonist-precipitated opioid detoxification under general anesthesia has received considerable publicity because it promises detoxification in several hours while the patient is unconscious and not experiencing withdrawal discomfort. A mixture of medications has been used, but morbidity and mortality as reported in the lay press are unacceptable, with no demonstrated advantage in long-term outcome (Collins et al., 2005).
Long-Term Management.
If patients are simply discharged from the hospital after withdrawal from opioids, there is a high probability of a quick return to compulsive opioid use. Addiction is a chronic disorder that requires long-term treatment. Numerous factors influence relapse. One factor is that the withdrawal syndrome does not end in 5-7 days. There are subtle signs and symptoms often called the protracted withdrawal syndrome (Table 24–7) that persist for up to 6 months.
 |
Table 24–7 Characteristics of Opioid Withdrawal
Physiological measures tend to oscillate as though a new set point were being established; during this phase, outpatient drug-free treatment has a low probability of success, even when the patient has received intensive prior treatment while protected from relapse in a residential program.
The most successful treatment for heroin addiction consists of stabilization on methadone in accordance with state and federal regulations. Patients who relapse repeatedly during drug-free treatment can be transferred directly to methadone without requiring detoxification. The dose of methadone must be sufficient to prevent withdrawal symptoms for at least 24 hours. The introduction of buprenorphine, a partial agonist at μ opioid receptors (Chapter 18), represents a major change in the treatment of opiate addiction. This drug produces minimal withdrawal symptoms when discontinued and has a low potential for overdose, a long duration of action, and the ability to block heroin effects. Treatment can take place in a qualified physician’s private office rather than in a special center, as required for methadone. When taken sublingually, buprenorphine (SUBUTEX) is active, but it also has the potential to be dissolved and injected (abused). A buprenorphine-naloxone combination (SUBOXONE) is also available. When taken orally (sublingually), the naloxone moiety is not effective, but if the patient abuses the medication by injecting, the naloxone will block or diminish the subjective high that could be produced by buprenorphine alone.
Agonist or Partial-Agonist Maintenance. Patients receiving methadone or buprenorphine will not experience the ups and downs produced by heroin (Figure 24–4). Drug craving diminishes and may disappear. Neuroendocrine rhythms eventually are restored (Kreek et al., 2002). Because of cross-tolerance (from methadone to heroin), patients who inject street heroin report a reduced effect from usual heroin doses. This cross-tolerance effect is dose-related, so higher methadone maintenance doses result in less illicit opioid use, as determined by random urine testing. Buprenorphine, as a partial agonist, has a ceiling effect at ∼16 mg of the sublingual tablet equaling no more than 60 mg methadone. If the patient has a higher level of physical dependence, a full agonist (methadone) must be used. Patients become tolerant to the sedating effects of methadone and can attend school or function in a job. Opioids also have a persistent, mild, stimulating effect noticeable after tolerance to the sedating effect, such that reaction time is quicker and vigilance is increased while on a stable dose of methadone.
Antagonist Treatment. Another pharmacological option is opioid antagonist treatment. Naltrexone (REVIA, others; Chapter 18) is an antagonist with a high affinity for the μ opioid receptor (MOR); it will competitively block the effects of heroin or other MOR agonists. Naltrexone has almost no agonist effects of its own and will not satisfy craving or relieve protracted withdrawal symptoms. For these reasons, naltrexone treatment does not appeal to the average heroin addict, but it can be used after detoxification for patients with high motivation to remain opioid-free. Physicians, nurses, and pharmacists who have frequent access to opioid drugs make excellent candidates for this treatment approach. A depot formulation of naltrexone that provides 30 days of medication after a single injection (VIVITROL) has been approved for the treatment of alcoholism. This formulation eliminates the necessity of daily pill-taking and prevent relapse when the recently detoxified patient leaves a protected environment.
|
|
|
|
BARBITURIC ACID (named Barbital for St. Barbara) was first synthesized from urea and malonic acid in 1863 by the German chemist Johann Adolf von Baeyer. Its properties were researched and reported four years later by two of his students.
Barbiturates and Older Sedatives. The use of barbiturates and older non-benzodiazepine sedating medications (e.g., meprobamate, glutethimide, chloral hydrate) has declined greatly in recent years owing to the increased safety and to the efficacy of the benzodiazepines and the newer agents zolpidem, eszopiclone, zaleplon, and ramelteon . Abuse problems with barbiturates resemble those seen with benzodiazepines in many ways.
Treatment of abuse and addiction should be handled similarly to interventions for the abuse of alcohol and benzodiazepines. Because drugs in this category frequently are prescribed as hypnotics for patients complaining of insomnia, physicians should be aware of the problems that can develop when the hypnotic agent is withdrawn. Insomnia rarely should be treated with medication as a primary disorder except when produced by short-term stressful situations. Insomnia often is a symptom of an underlying chronic problem, such as depression or respiratory dysfunction, or may be due simply to a change in sleep requirements with age. Prescription of sedative medications, however, can change the physiology of sleep with subsequent tolerance to these medication effects. When the sedative is stopped, there is a rebound effect with worsened insomnia. This medication-induced insomnia requires detoxification by gradual dose reduction.
This Webpage was created for a workshop held at Saint Andrew's Abbey, Valyermo, California in 2002