
HUMAN EMBRYONIC STEM CELLS
Human embryonic stem cells (hESCs) are nature’s master stem cells. They
are a self‑renewing source for the scalable manufacturing of functional
replacement cells for every tissue and organ in the body. The hESCs with
which Geron works were derived from surplus
in vitro fertilized embryos originally created as part of an in vitro
fertilization (IVF) procedure. The embryos, which would otherwise
have been destroyed, were donated for research by the parental donors
under informed consent. The hESC line that is used to produce GRNOPC1 is
the H1 line. Studies using this line qualify for U.S. federal research
funding, although no federal funding was received for the development of
the product or to support the clinical trial.
hESCs have two characteristics that make them different from other
naturally occurring stem cells. First, they are immortal – they express
the enzyme telomerase that enables the cells to divide endlessly in
tissue culture. This allows scalable manufacturing of therapeutic cells
derived from a master cell bank. Second, hESCs have the ability to
differentiate into any of the more than 200 functional, specialized
cells that make up the tissues and organs of the human body. Geron
scientists have learned how to grow undifferentiated hESCs under
carefully defined conditions, enabling them to be numerically expanded
to form large cell banks (hundreds of vials of frozen undifferentiated
hESCs) that serve as uniform starting material for manufacturing
procedures that convert the undifferentiated hESCs into functional
therapeutic cells. Geron scientists have learned how to manufacture
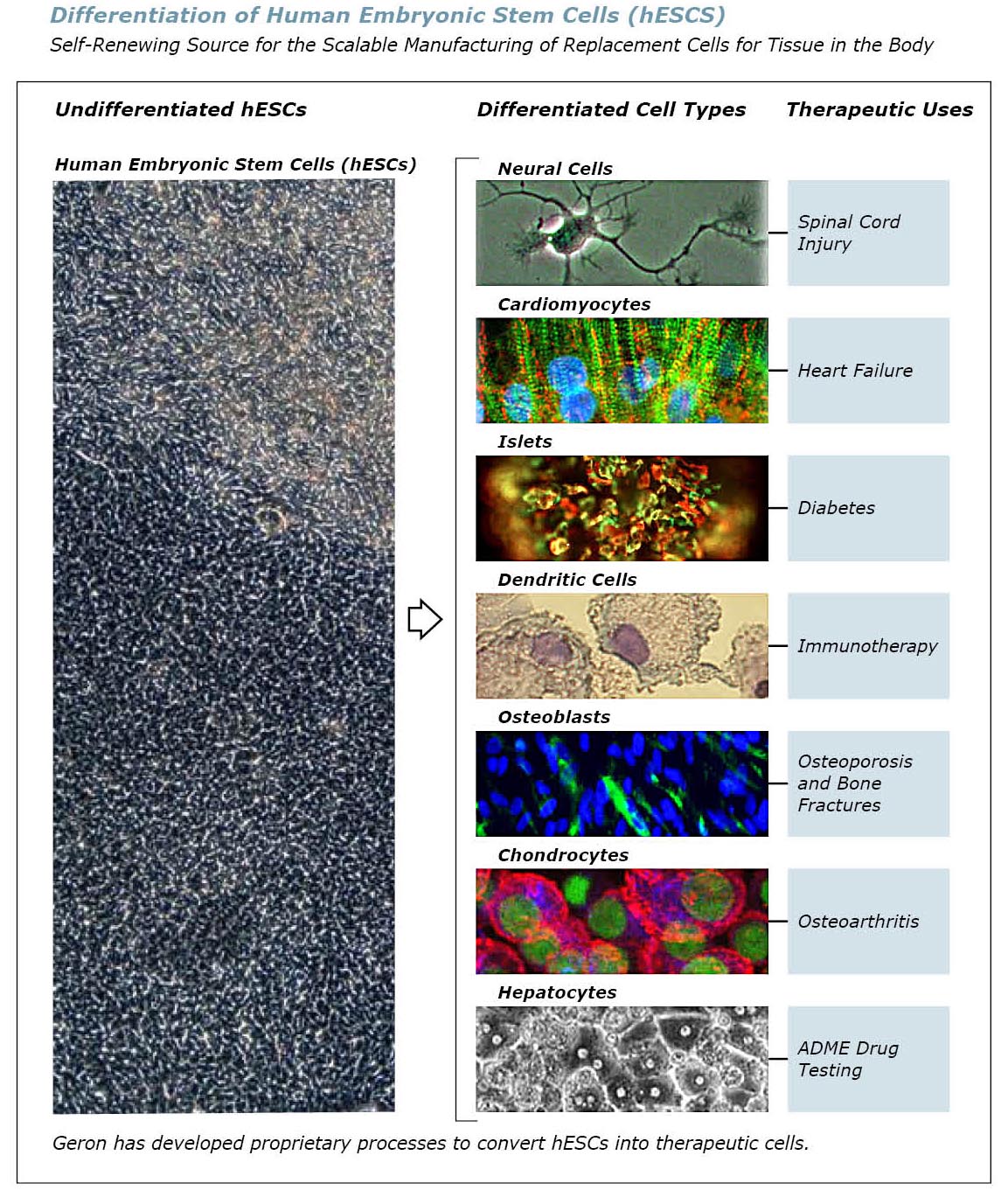
seven different types of functional cells from hESCs:
- neural cells to treat chronic degenerative diseases of the nervous system;
- cardiomyocytes for the treatment of congestive heart failure and myocardial infarction;
- islets for the treatment of diabetes;
- chondrocytes for the treatment of osteoarthritis;
- hepatocytes for ADME drug testing;
- dendritic cells cells for immunotherapy for cancer and infectious diseases; and
- osteoblasts for the treatment of osteoporosis and bone fractures.
These functional cells are produced from hESCs by specific processes in
which the frozen, banked hESCs are thawed, numerically expanded in their
undifferentiated state and then treated with specific biologicals and
growth factors to induce them to differentiate into specific functional
cell types for therapeutic use. Each cell type requires a unique
manufacturing “recipe.” All of the differentiated cells produced by the
manufacturing process are normal, healthy cells that have not been
genetically modified and which have characteristics similar to the
equivalent “natural” cell type present in the body.
Continue to Section 2: Oligodendrocyte Progenitor Cells (GRNOPC1) »
|